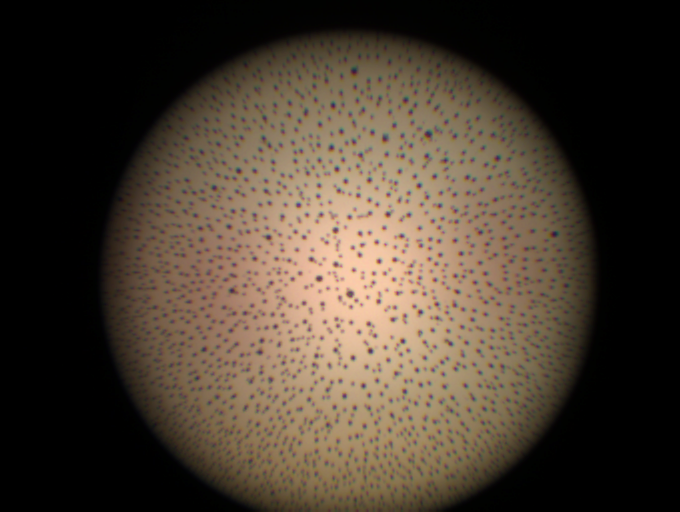
Endoscopes are vital medical devices that enable doctors to diagnose and treat a variety of conditions by providing a clear, internal view of a patient's body. However, like any medical device, endoscopes are subject to wear and tear, and over time they may need to be repaired or replaced.
To ensure the safety and effectiveness of endoscopes, manufacturers are required to conduct post-market clinical analysis (PMCA) of their products as part of the MDR .
We're specifically pointing to articles 83, 84, 85 and 86 of the European MDR 2017/745.
This involves collecting data on the performance and reliability of endoscopes in the field, and using this data to identify potential issues and improve the design of future endoscopes.
One way that endoscope manufacturers can improve their PMCA efforts is by using the data and image repository that endoscopemanager aggregated over almost 10 years of being in use. EndoscopeManager is a cloud software tool that helps track and manage endoscope quality data and gives hospitals more predictability and traceability.
With endoscopemanager.com, manufacturers can easily collect and organize anonimzed data on endoscope usage, performance, and maintenance. This data can then be used to identify trends and patterns, and to pinpoint areas where endoscope performance may be lacking.
Furthermore, Dovideq has aggregated over 2 million images of the lens structure of over 90 brands of rigid endoscopes.
Endoscopemanager can help manufacturers track endoscopes in use, the frequency of their use, and link that with their own data, such as the types of procedures they are being used for. This information can be used to determine the average lifespan of an endoscope, and to identify potential areas of risk where endoscopes may be failing prematurely.

In addition to tracking endoscope usage and performance, endoscopemanager can also help manufacturers monitor the maintenance and repair of their endoscopes. This can be especially useful for identifying potential problems with endoscope design, such as parts that are prone to failure or difficulty in cleaning. By identifying and addressing these issues, manufacturers can improve the reliability and safety of their endoscopes.
Overall, endoscopemanager is a valuable tool for endoscope manufacturers looking to improve their PMCA efforts. By providing easy access to endoscope data, an endoscopemanager can help manufacturers identify trends and patterns, and pinpoint areas where endoscope performance may be lacking. This information can then be used to make informed decisions about endoscope design and maintenance, ultimately improving the safety and effectiveness of endoscopes for patients.