
Endoscopes are vital medical devices that enable doctors to diagnose and treat a variety of conditions by providing a clear, internal view of a patient's body. However, like any medical device, endoscopes are subject to wear and tear, and over time they may need to be repaired or replaced.
To ensure the safety and effectiveness of endoscopes, manufacturers are required to conduct post-market clinical analysis (PMCA) of their products. This involves collecting data on the performance and reliability of endoscopes in the field, and using this data to identify potential issues and improve the design of future endoscopes.
One way that endoscope manufacturers can improve their PMCA efforts is by using an endoscopemanager.
Endoscopemanager is a software platform that can help Original Equipment Manufacturers (OEMs) and repair companies improve and speed up their logistics supply chain.
By using endoscopemanager, OEMs and repair companies can track the repair and exchange cycle of endoscopic devices, ensuring that they are delivered to hospitals in a timely and efficient manner by providing real-time tracking and visibility of endoscopic devices.
This means that OEMs and repair companies can quickly identify where a particular device is in the supply chain, allowing them to make informed decisions about how to best manage their inventory and logistics and to better plan their surgical procedures and improve their overall efficiency.
This is particularly useful for hospitals that regularly use endoscopic devices and need to ensure that they have enough of them on hand to meet their surgical needs.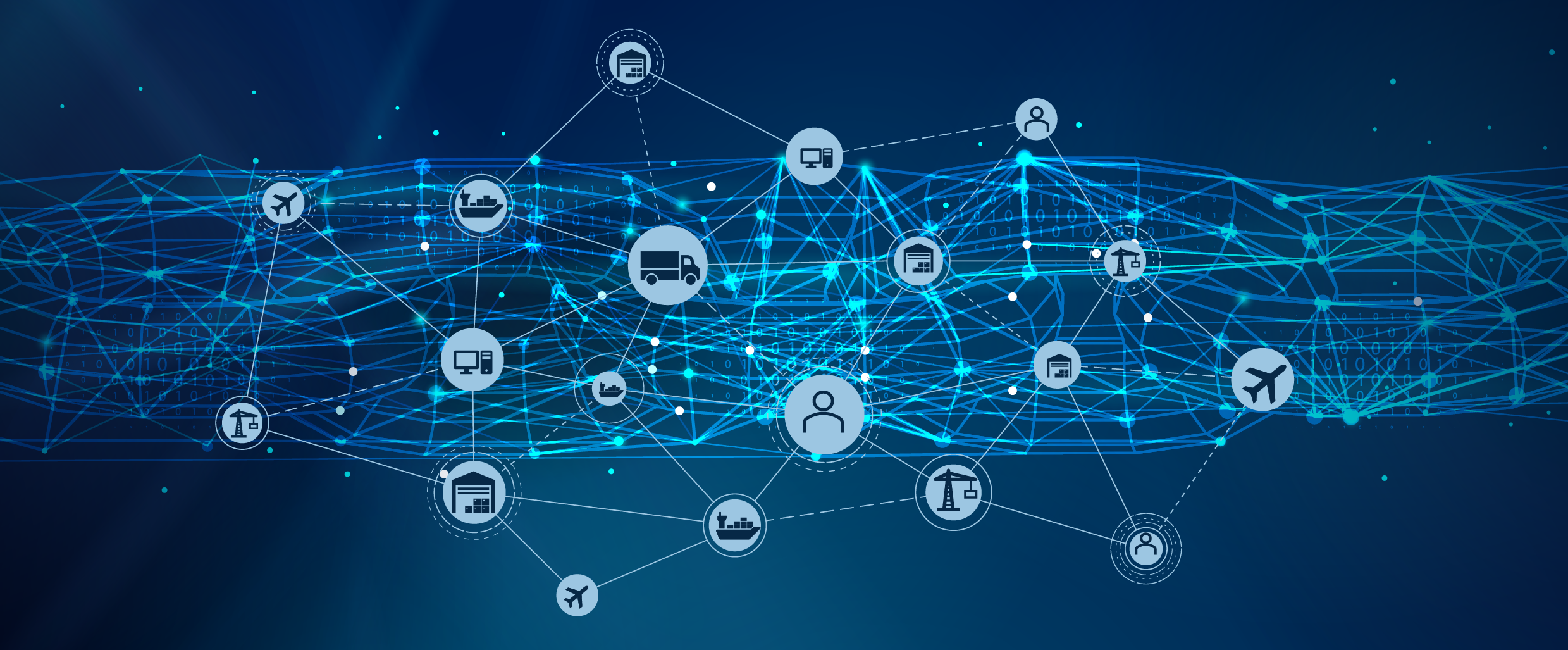
When it comes to quality assurance and control, endoscopemanager can play a critical role in ensuring that endoscopic devices are consistently of high quality.
By tracking the repair and exchange cycle of these devices, OEMs and repair companies can identify any potential issues and take steps to address them before they become major problems.
This helps to ensure that hospitals are consistently provided with endoscopic devices that are safe and effective for use in surgical procedures.
In addition, this can be useful for identifying potential problems with endoscope design, such as parts that are prone to failure or difficulty in cleaning.
By identifying and addressing these issues, manufacturers can improve the reliability and safety of their endoscopes.
Another important benefit of endoscopemanager is that it can help to improve the repair and exchange cycle of endoscopic devices.
By tracking the status of these devices in real time, OEMs and repair companies can easily identify when a device needs to be repaired or exchanged, allowing them to take action and ensure that the device is back in the hands of the hospital as soon as possible.
This can help to reduce downtime and improve the overall efficiency of the repair and exchange process.
Overall, endoscopemanager is a valuable tool for OEMs, repair companies, and hospitals that use endoscopic devices.
By providing real-time tracking and visibility, endoscopemanager can help to improve the logistics supply chain and ensure that endoscopic devices are consistently of high quality.
It can also help to improve the repair and exchange cycle, reducing downtime and improving the overall efficiency of the process.


