Comparing Endoscope Testing Solutions: How Dovideq’s LightControl Stands Out
Since the advent of endoscopes in healthcare, surgeons have struggled with their complexity and the ensuing headaches caused by deteriorating quality and visibility. Damage to lenses or fibers often results in poor image quality or inadequate illumination, exacerbating these issues.
Within modern hospitals, the Central Sterile Services Department (CSSD) is responsible for ensuring the quality and suitability of rigid endoscopes. However, inspection methods vary widely, ranging from manual visual checks to fully automated systems capable of autonomous testing and registration.
It’s crucial to recognize the disparity between evaluating endoscope quality in a well-lit room through direct eyepiece assessment and its actual use during surgical procedures, where a camera mounted on the endoscope magnifies any defects in the device along with the image produced.
In this whitepaper, we will contrast Dovideq’s LightControl with other inspection tools for endoscopes, which offer varying levels of complexity and automation.
Download this blog as a whitepaper

About LightControl
LightControl stands as Dovideq’s comprehensive solution for verifying the quality of rigid endoscopes. It meticulously evaluates endoscopes across six carefully chosen parameters, providing valuable insights into the endoscope’s quality and suitability for use.
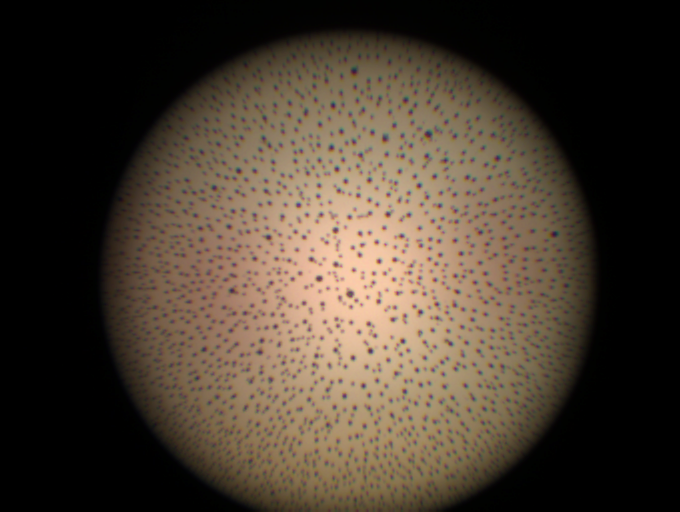
Employing a sophisticated camera system, LightControl captures snapshots of each lens as well as the internal patterns of the testing sphere, which replicates the image a surgeon would perceive during a procedure. This rigorous examination assesses light transmission, color correctness, focus, lens fractures, and the presence of particles in the internals.
In addition to the camera system, LightControl utilizes a sensor attached to the light post to gauge the quality of the fiber package by measuring light transmission.
By combining these measurements, LightControl passes or fails the endoscope based on user-defined thresholds for each parameter. The results of each test are then archived in EndoscopeManager.com under the respective endoscope’s serial number.
EndoscopeManager is Dovideq’s own cloud platform where users can view their previous test results sorted by serial number, automatically generate PDF reports, and much more.
Manual Inspection
Manual inspection demands a skilled and experienced individual to conduct a visual assessment of the endoscope’s exterior, transmitted image quality, and fiber package. Some departments augment this process with magnifying glasses and dedicated light sources to inspect fibers more closely. However, a study done in 2016 at the Hospices Civil de Lyon concluded that this method of inspection fails to identify around 80% of defects in a rigid endoscope.

Dale 301 / EndoScan™
Devices such as the Dale 301 and EndoScan aim to alleviate the challenges associated with manual endoscope inspection. They facilitate the adjustment of focal points to evaluate individual lenses, not unlike LightControl’s internal image capture process.
However, the key disparity lies in automation: while LightControl’s camera system automatically focuses, captures images, and archives them in the cloud, handheld inspection tools like the Dale 301 and EndoScan merely aid the manual inspection process. These devices can of course be used in combination with a camera for documentation, but this is not their primary purpose.

EndoCam Pro
Contrary to tools focused on internal inspection, the EndoCam Pro concentrates on assessing image quality. By providing a live view of the image on a small screen, with the option to connect to larger displays, it bridges the gap between inspection and surgical use. However, unlike LightControl, it lacks insight into the internal state of the endoscope and the quality of the fiber bundle.
Any images or videos taken also have to be retrieved from an SD card instead of being automatically uploaded and sorted by serial number.

MedZense LG-20 E
Shifting attention from lenses to fiber packages, the MedZense LG-20 E evaluates an essential aspect of endoscope functionality: lighting. By measuring light transmission and color spectrum performance, it ensures adequate illumination for surgical procedures. To test endoscopes, however, the device must first be calibrated with the light guide cable used before testing the endoscope.
While it is a great tool, it offers no insight into image quality and lens condition, focusing solely on the fiber package while the assessment of the image quality is up to the operator or a different inspection tool.
Conclusion
In conclusion, the landscape of endoscope inspection tools offers a spectrum of options, each with its unique capabilities and limitations. While manual inspection methods rely on skilled individuals to assess endoscope quality, advanced technologies like Dovideq’s LightControl revolutionize the process with comprehensive automation and precise evaluation metrics.
LightControl’s sophisticated camera system and meticulous testing parameters set a new standard for endoscope quality assurance. By capturing detailed snapshots and analyzing key aspects such as light transmission, color accuracy, and lens integrity, it provides invaluable insights into endoscope performance, ensuring optimal functionality for surgical procedures.
While alternative tools like the Dale 301, EndoScan, EndoCam Pro, and MedZense LG-20 E offer valuable contributions to the inspection process, they often fall short in terms of automation, comprehensive evaluation metrics, or insights into either fiber bundle quality or image quality.
Ultimately, the choice of inspection tool depends on the specific needs and priorities of healthcare facilities. However, for those seeking a comprehensive, efficient, and reliable solution for endoscope quality assurance, Dovideq’s LightControl emerges as a frontrunner, setting a new benchmark for endoscope inspection excellence.
[Download the full whitepaper here]