In the realm of healthcare, the safe and effective reuse of medical devices, particularly endoscopes, is critical. ISO 17664, an internationally recognized standard, plays a pivotal role in guiding hospitals on proper sterilization processes and maintenance procedures for reusable endoscopes.
Good to know: ISO 17664 is a harmonized norm in the European MDR 2017/745.
This blog explores the significance of ISO 17664 for hospitals, focusing on the reuse of endoscopes and the crucial elements of maintenance, inspection, and testing.
ISO 17664 titled "Sterilization of medical devices—Information to be provided by the manufacturer for the processing of resterilizable medical devices," is a comprehensive guideline that assists manufacturers in providing essential information to end-users regarding the proper sterilization and reprocessing of medical devices.
This standard serves as a crucial tool in maintaining patient safety and preventing the transmission of infections associated with inadequately reprocessed endoscopes.
Maintenance, Inspection, and Testing is an integral part of the ISO plays a vital role in ensuring the safety and efficacy of reusable endoscopes.
ISO 17664 emphasizes the significance of establishing robust protocols for these processes. Regular testing procedures help identify and rectify potential issues promptly, ensuring optimal performance of the endoscopes during subsequent procedures.
Thorough inspections and testing, following the guidelines within ISO 17664, allow hospitals to assess the effectiveness of their reprocessing procedures and validate the quality of sterilization achieved.
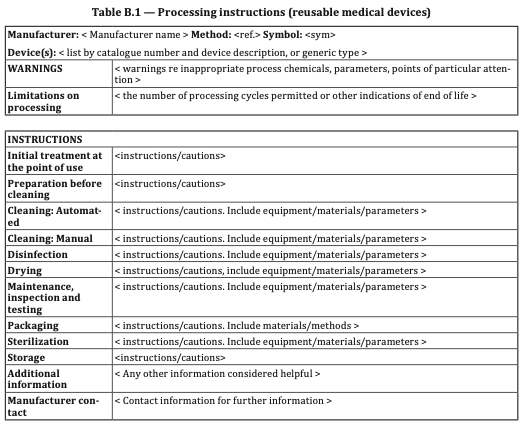
Connecting Maintenance, Inspection, and Testing to Table B.1:
Table B.1, an integral part of ISO 17664, provides hospitals with valuable information pertaining to each phase of the reprocessing process for medical devices. In the context of endoscope reuse, Table B.1 serves as a comprehensive resource, covering essential aspects such as cleaning, disinfection, sterilization, and storage.
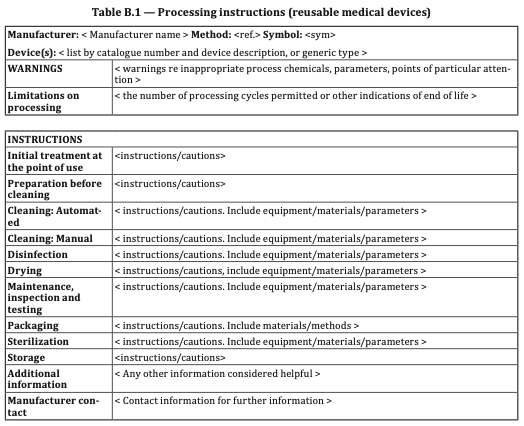
This table enables hospitals to establish standardized protocols based on clear information provided by their manufacturers, aligning maintenance, inspection, and testing procedures with the specific requirements outlined in ISO 17664. By referencing Table B.1, hospitals can ensure that they are implementing the necessary measures at each reprocessing phase, reducing the risks associated with inadequate endoscope reprocessing.
Testing plays a crucial role in the reuse of endoscopes and is an essential aspect emphasized by ISO 17664. It allows hospitals to assess the effectiveness of their reprocessing procedures and validate the quality of sterilization achieved. Through testing, hospitals can ensure that the endoscopes have undergone proper cleaning, disinfection, and sterilization, thereby minimizing the risk of infection transmission to patients. Additionally, testing helps identify any potential issues or failures in the reprocessing process, allowing hospitals to take corrective measures promptly.
The importance of Original Equipment Manufacturers (OEMs) supplying parameters, instructions, and cautions to hospitals cannot be overstated.
OEMs possess in-depth knowledge about the design and functionality of the endoscopes they manufacture. By providing comprehensive information, OEMs enable hospitals to understand the specific requirements for reprocessing their endoscopes effectively. This includes parameters such as recommended sterilization methods, compatible disinfectants, and appropriate cycles for cleaning and sterilization equipment. Clear instructions and cautions from OEMs help hospitals adhere to standardized practices and ensure that the endoscopes are processed in a manner that maintains their integrity and functionality. Ultimately, the collaboration between OEMs and hospitals, with the provision of crucial information, facilitates the safe and effective reuse of endoscopes, safeguarding patient well-being.
ISO 17664 serves as a guiding light for hospitals, addressing the challenges related to the safe reuse of endoscopes. By adhering to the guidelines set forth in this standard, hospitals can establish robust protocols for the maintenance, inspection, and testing of endoscopes. These practices, connected to the relevant information provided in Table B.1, enable hospitals to meet the highest quality and safety standards while mitigating the risks associated with substandard reprocessing. Ultimately, ISO 17664 empowers hospitals to prioritize patient safety and deliver healthcare services with confidence in the reusable endoscope processes.