Endoscopes have become so important in the operating room that the risk and cost of faulty ones are no longer acceptable. Because of increased use of endoscopes in hospitals, wear and tear on the devices is having an increasing impact. In particular, the sterilization process, usually with steam at 135°c, is a strain.
If an endoscope falls on the floor, the viewing angle becomes dislocated or moisture and contaminants can enter the light path and disrupt the image. Research in Dutch operating rooms shows that 4% of endoscopes do not function properly. Abroad, the percentage of non-functioning endoscopes is much higher, up to 10%.
The surgical team only finds out that an endoscopes is faulty during an operation when the patient is already under anesthesia. If a replacement can be arranged at all, it means a delay, which adds cost and is always a risk to the wellbeing of the patient.If a replacement cannot be arranged for the endoscope, the entire procedure must be called off or the patient must still undergo traditional surgery. Most hospitals have a "spare endoscope" lying around. This is dead capital and can be avoided.
How it started
With that problem, the founder of Dovideq was approached in 2009. It turned out that there was no instrument to measure endoscopes in the hospital on a regular basis.
They go to the supplier for inspection from time to time, but that's only once a year at best.
Medical professionals wanted to integrate the inspection into the standard operating procedure of the hospital. They began thinking about a solution and after a year of simmering, it hit experimentation in 2010 and the following year, a prototype was ready and Dovideq Medical was founded.
In addition, Dovideq had to go through a clinical validation. This was done together with the UMC Utrecht. In addition, several hospitals purchased a pre-production model, which were soon exchanged for the final model.
Dovideq had an appealing solution. In 2012, the jury of the 'Twente' Young Technology Award also thought so, as did the jury of Shell Livewire award in 2012, beating four other finalists. It also won the MKB Export Award in 2013. More importantly: the hospitals were particularly interested.
All that time, the company paid for the development with a combination of its own capital and subsidies such as innovation vouchers. When working with the UMC Utrecht, a substantial subsidy became available.
At Dovideq, we think properly testing endoscopes is a vital step in the process, which should be easy and provide all the information necessary to mae sure you're dealing with safe equipment. Because quality for a patient comes first! Cost savings for the OR team and sterilization department are important as well.
Operating theatre and CSSD will gain efficiency improvements with our products, that will reduced costs, making the investment in our products worthwile.
The market launch for ScopeControl was financed in 2015 through crowdfunding. Within 3 days the 150 thousand Euros were funded. After that, several investments were done by different institutional financiers.
Customers from all over the world came to Dovideq for this prize winning innovation, mainly because ScopeControl has patented technology, that is truly beneficial to any department working with reuse of rigid endoscopes.
Serious business
"After an operation, all instruments in the hospital go to a central sterilization department. We developed the Scopecontrol for this purpose, a device that does all the optical tests for a rigid endoscope," explains Chielant de Wit, CEO.
'"You perform them every time before sterilization. By testing in the normal workflow, we ensure that the chance of an endoscope being defective on the OR is practically eliminated."
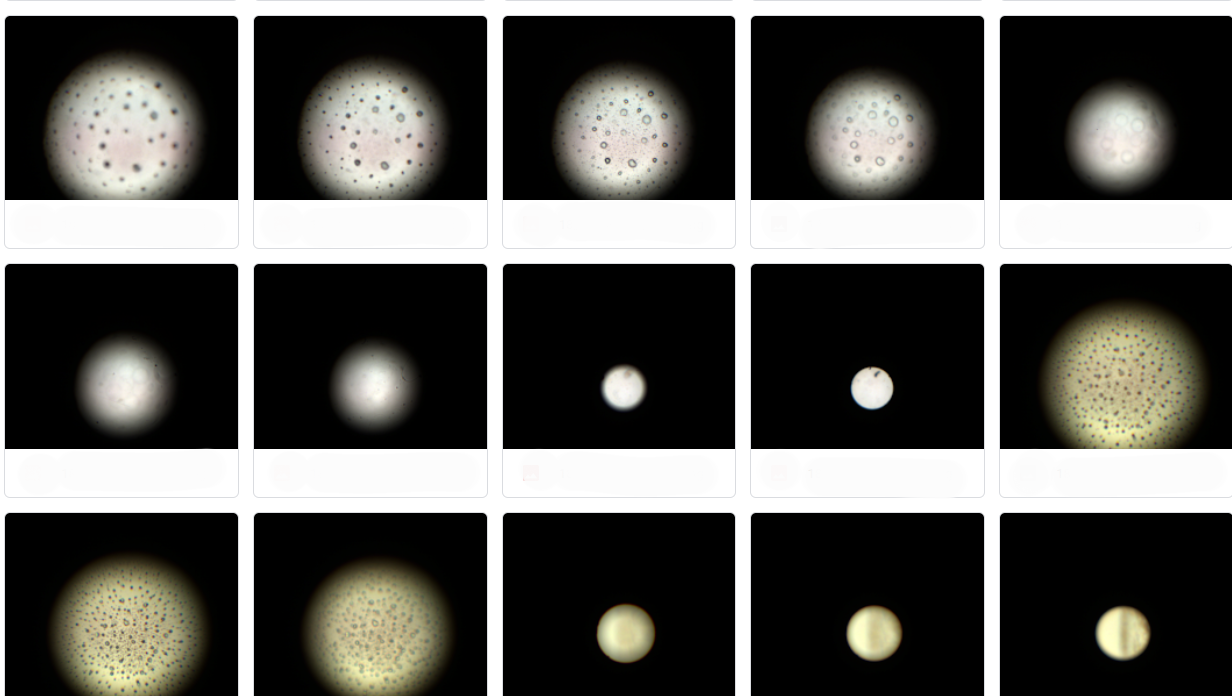
ScopeControl is based on image processing. When the test starts, the endoscope is inserted into a sphere with a reference pattern on the inside, say an artificial abdominal cavity. On the other side of the endoscope, an image sensor picks up the image through a lens system. 'Depending on the angle of the endoscope, you should see certain markers. With image analysis we check if those angles are right, if the markers are sharp and the colors are right.'
'You just put an endoscope in it, press start and in no-time it does all the optical tests,' de Wit describes. The automation in the device takes care of grabbing the instrument and sliding the reference bulb over it at the right rate; a (touch) screen displays the results. 'It also records all the data from each endoscope for evidence afterwards, 'We've built a complete management system, so you can see in advance when an endoscope will break down. We also offer the cloud service for that.'
Current Situation
Currently, there is legislation, amongst which the European Medical Device Regulation, which requires hospitals to document and store functional tests of endoscopes.
Actually, all (supra)national regulatory authorities require quality control of endoscopes before each reuse. In addition, a record must be kept of each and every reuse, making Dovideq's product line up more essential for the medical profession.
With our products, defects are immediately detected and an endoscope can be offered for repair with clear indicators, even automating the process between repair firm and hospital. Why lose so much time when a robot can do it in half for almost no cost?
In 2020 Dovideq was strategically bought by Con-Vergence Industry BV which is specialized in engineering and assembling high-end test equipment and offer software and maintenance services as well.
In 2021 Dovideq raised capital from several informal investors to increase its potential to the fullest.
The Research & Development department is still in full swing and currently working hard on new innovations.
In the meantime, there are stable exports to the Middle East, Latin America, Asia, Europe, Great Britain, Canada and the United States.
However, Dovideq is always on the look out for new partners.