Sterilization processes are important to all medical procedures.
The idea and need to keep out harmful micro organisms from contaminating medical procedures cannot be overemphasized.
Many times this burden falls directly on the Sterilization Department manager. Doing it well directly influences the sterilization efficiency. It’s an all or none principle –you either get it right for once, increase efficiency to cut price, or the safety of your medical equipment becomes compromised. You might not want to go down that path.
In this review, we discuss how best to get your automated endoscopy done with zero or no error margins. You might also consider getting a well-research digital management tool for your collection of medical devices.
Automated Endoscope Testing
Verifying the quality of your endoscope helps you better safeguard patients’’ health. For several reasons, you might not want to do this manually. In addition to cumbersomeness and time wastage, the manual endoscope testing method also presents a high risk of error. The FDA’s stance on the quality of reusable medical devices –including rigid endoscopes –is clear. Rigid endoscopes are expected to be tested for quality, updated, and decontaminated for follow-up usage.
As a Sterilization manager, you might want to consider endoscope testing processes featuring modern innovation in equipment validation and quality assurance. The general recommendation currently acceptable to many healthcare stakeholders prescribes an in-process quality check and periodic testing for equipment validation. Hospitals, endoscope manufacturers, and endoscopy facilities have transitioned to modern methods that swiftly reduce error, cut costs, get the job done quickly, and most importantly, improve the efficiency of endoscope testing.
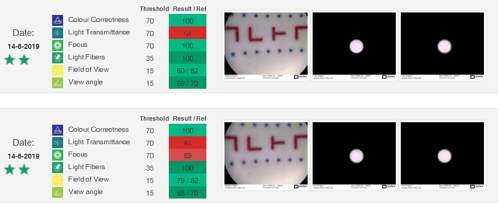
Our ScopeControl endoscope tester is designed to verify the quality of rigid endoscopes. The validation and quality check process of this technology complies with the global standard on equipment safety. To properly cater to your quality assurance needs, ScopeControl gives you 6 fully automated test and measurement options. Every test on this control plan directly verifies the quality of your endoscope’s elements under preset specifications and time.

Dump Manual Quality Checks, Use ScopeControl
Sterilization Managers that need a trustworthy update on equipment safety can go a long way to get it. ScopeControl is designed to give the best collection of data that directly reflect equipment safety and quality. This technology verifies the viewing angle, comparing it with the manufacturer’s specifications.
If the angle of view is altered, a Surgeon’s in-use endoscopic viewing assessment can be faulty, increasing the risk of surgical errors. ScopeControl also tests the endoscope's light fibers, light transmission efficiency, focus, and color balance. The testing procedures are fully automated and designed to compare and validate the manufacturer’s reference standards.
Get the Best Digital Management Tool for your Medical Devices
If you are serious about the efficiency of your medical equipment, you might want to consider a digital management tool. The general idea is to follow up on the quality process ad validation measurement of this equipment. Since the outcome of excellent healthcare services depends significantly on the quality of medical devices available, next-generation digital management tools are your best bet.
EndoScopeManager and Your Medical Equipment
This product gives you a detailed assessment of the functional status of your endoscope. From quality monitoring to fully automated logistics and repairs, this product is designed to guide your decisions on device optimization. In hospitals, you can easily compare endoscope brands, check for repairs and manage equipment handling.
EndoscopeManager for the endoscope manufacturer offers a detailed digital overview of the production process. You can easily compare the compliance with production standards and the reference quality specifications. The tracking option also helps you follow and manage your endoscope remotely.
Sterilization department managers and product quality assurance teams can automatically collect repair reports and check for the functional integrity of their endoscope. EndoscopeManager is specially built to get you through. Not only do you save your job, but you also improve the operational efficiency of your endoscopes.
Final Thoughts
Automated endoscope testing and digital management tools are the next-gen approaches in endoscope quality assurance. For in-process use and storage, you can easily optimize the functionality of your rigid endoscopes with the technologies highlighted in this post. These technologies are fully automated and can help the surgeon avoid errors during endoscopy procedures. Manual testing and management processes can increase the chances of these errors and adversely affect the outcome of surgery.
REFERENCES




